During a cat reformer regen we are required to monitor the pH and alkalinity of a wash water solution in the process.
This year we had issues with controlling the alkalinity and we used so much of our neutralising solution, we ran out.
This post will explain the background and detail what went wrong.
Background
What is a Cat Reformer?
A cat reformer is a process unit within a refinery that takes low octane feed and produces a high octane 'reformate' that can be blended with other streams to produce petrol.
What is a regen?
While the unit is online, coke builds up on the surface of the catalyst. This reduces the activity of the catalyst and stops it working.
About every 18 months, the catalyst is regenerated and returned to its fresh state. The process is stopped and all the hydrogen and hydrocarbons are removed. The system is started back up with nitrogen and a small quantity of air is added to allow a controlled 'burn' to remove the coke.
What has this got to do with pH?
The catalyst contains a small quantity of chloride. During the burn, water is produced and this stripps the chloride from the catalyst. When the water and chloride condense together, this produces hydrochloric acid which, if left, would eat away at the fin-fans and pipework.
What we do to avoid this is circulate a 'wash water' solution around the parts of the circuit that may be affected by the acid and add soda ash (sodium carbonate) to neutralise any acid that is produced in the process.
We take samples of the wash solution roughly every hour and based on the results, amend the soda ash addition rate.
Process detail
pH vs alkalinity
When we analyse the sample of wash water, we test for both the pH and the alkalinity of the solution.
The pH is effectively the concentration of acid ions (less than 7 is acidic, greater than 7 is alkaline). As far as we are concerned, we need to ensure the solution does not go too acidic and aim to keep it above 6.5.
However pH is a poor measurement to use because while it tells you where you are at that moment, it doesn't actually tell you how far you are from the danger point. That is, how much more acid can I add to the wash water solution before it goes bad.
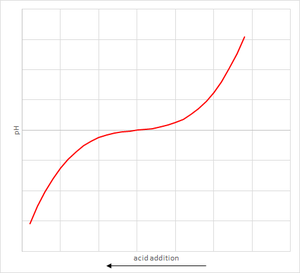
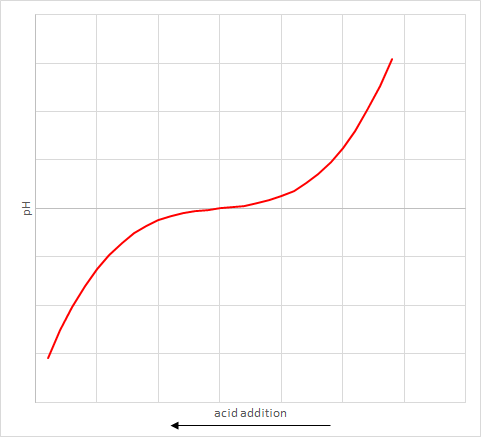
The reason is that adding a fixed quantity of acid can change the pH by different amounts depending on what the starting pH is. It is not a linear response. An example graph of how the pH varies with quantity of acid added is shown. Note that the size of flat bit in the middle will vary with each solution.
The actual measurement of acid required to shift the pH to the trigger point is called the alkalinity. This is the main measurement used to determine the quantity of soda ash to inject.
How do you test the alkalinity?
To measure the alkalinity of the wash water, we carry out titrations on the sample. This is where a small measured quantity (2ml) of the wash water is placed in a conical flask and acid is slowly added until the pH drops below a set point.
The quantity of acid added is then used to calculate what the concentration of a of sodium hydroxide solution would be to have the same effect. This is the alkalinity.
To determine when the pH drops below the required value, an indicator is used. This is a compound that has a very distinct change in colour when the pH changes at value, known as the end point. A few drops of indicator are added to the solution before adding the acid. Acid is continually added until the solution changes colour, indicating the end point has been reached and the total volume of acid added is then used in the calculation.
The event
What happened?
Ideally we operate with an alkalinity of 1.5 to 2.0 (units %wt NaOH equivalent) and this year we struggled to maintain anything above 0.2 even while maxing out our soda ash injection.
As a result, we used far more soda ash than anticipated and managed to run out at 04:00 in the morning.
What went wrong?
It turns out there are two indicators that look similar however have different end points. The indicator we were supposed to use was Bromophenol blue with and end point of 3 to 4.6. Insted, we were using Bromothymol blue with a much higher pH end point, around 6 to 7.6.
As a result, the solution was changing colour during the flat part of the graph, instead of at the steep bit at the end. We actually had a really high alkalinity but were not measuring most of it. This lead us to increase our soda ash addition until it was at maximum and eventually it ran out.
Go Top« Viso: How to Split Lines
Dictionary Fail »




Comments
I would love to know what you think. To comment on this article, send me an email
No comments yet.